
The Manzanita legacy
The Manzanita tree is part of the California chaparral, a genre of native plants that survives brushfires and can thrive even in difficult environments.
History of non-dilutive funding
Manzanita Pharmaceuticals, Inc. was founded in May 2007 with data supported by DARPA in a prior corporate entity. Since founding, Manzanita Pharmaceuticals has been awarded three grants:
- Department of Defense ($992K - completed),
- National Cancer Institute SBIR Phase I ($288K - completed), and
- National Cancer Institute SBIR Phase II ($1,972K – current).
The company continues to pursue further non-dilutive funding opportunities.
Patent portfolio
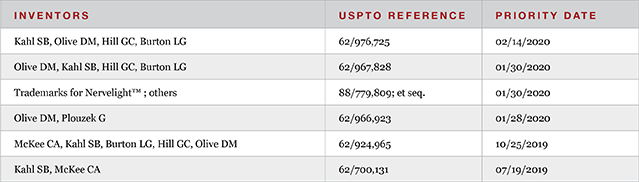
As of March 2020, the company had not licensed in any technology.
Current filed and provisional patents discuss Nervelight™, delivery to the posterior of the eye, anti-cancer conjugates, and anti-inflammatory agents.
Team
Management
- Constance McKee is co-founder and serves as President & CEO
- Stephen B. Kahl, PhD serves as Chief Scientific Officer, Executive Vice President & Principal Scientist
Constance McKee, President & CEO
Constance is co-founder, President and CEO of Manzanita Pharmaceuticals. She is a named co-inventor on three issued Manzanita patents and several current patent applications. She supports the PI Kahl as Project Manager for the SBIR Phase II grant and acted as co-PI for the Department of Defense grant completed in 2019. Since founding Manzanita Pharmaceuticals, Inc. in 2007, she has raised $3.3M in non-dilutive funding and about $350K in private angel investment.
Constance graduated with honors from Stanford University and earned an MBA from Yale University in 1986. In 1986-87 she was awarded a Bosch Fellowship in Germany which supported internships in corporate finance in Frankfurt, Germany. From 1990-1994 in Cambridge, England, she served Chief Executive of Cambridge Quantum Fund I (CQF) a seed venture fund at Cambridge University.
Since returning to the US in 1995, Constance advised or consulted to another 25 technology and life sciences start-ups. From 1996-2001 she worked as a contractor for Philips Electronics on over 20 M&A transactions, licensing agreements, intellectual property-based divestments, and venture capital assignments. From 2010-present, as i2 Grants Associates, LLC, she prepares federal and defense-related life science grants and has raised approximately $10.7M for third parties.
From 2003-2016, she created and then guest-taught the non-credit short course Business of Biotechnology at Yale University. From 2015-2019, Constance was a guest speaker at Entrepreneurship Boot Camp for Veterans with Disabilities at University of Connecticut. She is a fierce advocate for better cures and therapies to treat our Wounded Warriors and our Veterans.
Stephen B. Kahl, PhD, Chief Scientific Officer; Vice President & Principal Scientist
Dr. Stephen B. Kahl serves as the PI on Manzanita Pharmaceuticals’ NCI SBIR Phase II grant to develop a contrast agent, and recently served as PI on the company’s DOD grant to develop a non-opioid pain medication. Dr. Kahl previously served as PI on the company’s founding NCI SBIR Phase I grant, and was a Consultant to the company in support of a founding DARPA grant that successfully demonstrated company’s technology. He is an inventor on several of the issued Manzanita patents and on most patents currently in prosecution.
In 2011, Dr. Kahl retired as Vice Chair, Department of Pharmaceutical Chemistry, the University of California, San Francisco (UCSF) after serving in that position for over 20 years. He continues to teach and coordinate two pharmacology courses, Emeritus, in recall, and serves as a member and co-chair of the Pharmaceutical Sciences Pathway Steering Committee.
For the past 30 years, the focus of Dr. Kahl’s research was the design, synthesis, and in vitro and in vivo biological evaluation of potential new tumor targeted drugs for use in binary cancer therapies. These include boron neutron capture therapy, where the drug activating agent is thermal neutrons, and photodynamic therapy, where activation is by visible light.
At UCSF, Dr. Kahl investigated a variety of tumor-specific targeting strategies such as using low density lipoproteins (LDL) as drug carriers, the use of small peptides known as nuclear localization sequences for drug delivery to cellular nucleii, and other small peptide “zipcodes” identified by phage display for selective drug delivery to cell surface sites. One compound from the Kahl lab was brought from the initial synthesis of a few milligrams through small and large animal models and eventually into successful Phase 1 clinical trials for photodynamic therapy of malignant glioma. Unusually for an academic scientist, he is well versed in the translational challenges of bringing new drug entities from bench to bedside.
Dr. Kahl earned his BS from Duke University in Chemistry (1968), and his PhD from Indiana University in Inorganic Chemistry (1972). He completed a postdoc at the University of California, Berkeley in Chemistry and Physics (1972-1974), then spent eight years at Wellesley College. In 1983 he accepted a position at UCSF, where he maintained an active laboratory until his retirement in 2015. Now Emeritus, Dr. Kahl continues to teach at UCSF.
Board of Directors
The Board of Directors consists of six of our nine private investors: Constance McKee MBA, Thomas Knobel (Chairman), Robert Dalziel PhD, Robert Webb PhD, Gary Blair MD, and Steve Borst. Christopher J. Dunn MD PhD FCCP serves as an Observer, Board of Directors.
Thomas Knobel, Chair, Board of Directors
Mr. Knobel is Managing Partner for Global Main Streets Associates, LLC, providing business and technology consulting for corporate, government, and not-for-profit clients worldwide.
With footholds in business, academia, government, philanthropy, and investment, GMSA and its predecessor, PK Associates, has a 26-year history of successful engagements for large and small companies, start-ups, national laboratories, global foundations, national and international NGOs, and an investment bank.
Clients included Oak Ridge National Laboratories, the European Union, Dow Chemical, IBA (Brussels), The Bill and Melinda Gates Foundation, and Kent State Development Corporation.
Mr. Knobel spent 24 years as senior researcher and senior account manager for Dow Chemical, and later as Technical Director at E-BEAM Services. As a principal investigator he was granted 23 US and foreign patents.
Along the technical spectrum, GMSA’s Mr. Knobel offers specific expertise in several fields, focused on evaluations of new processes and products, business planning, and business development.
For six years Mr. Knobel volunteered as Executive Coach/Advisor at entrepreneurial boot-camps at Cornell and Syracuse Universities. He is an active private equity and angel investor. Mr. Knobel is Senior Advisor at NOHMs Technologies, Inc., an advanced battery company.
He holds BS degrees in Biology and Chemistry from the University of Houston. Mr. Knobel is a published author and an active public lecturer on topics including technology and energy.
Robert G. Dalziel PhD, Board Member; Chair, Scientific Advisory Board
Dr Dalziel serves as Chair, Scientific Advisory Board of Manzanita Pharmaceuticals. He joined the Asilomar team in 2003 as subaward Principal Investigator under a sponsored research agreement at the University of Edinburgh. His laboratory carried out the foundational in vivo studies showing analgesic activity of an injected “neurotrophin targeted glucocorticoid” conjugate.
Dr Dalziel is a Group Leader and Senior Lecturer in The Roslin Institute and the Royal (Dick) School of Veterinary Medicine at the University of Edinburgh. He gained his BSc (Honours) in Biochemistry from the University of Glasgow in 1980. He carried out his PhD studies on protein/DNA interactions in HSV-1 infected cells at the MRC Institute for Virology, graduating in 1984. He then worked at the Scripps Research Institute and in 1987 accepted a Faculty Position at the University of Edinburgh.
Following a Wellcome Trust lectureship in Molecular Biology (1987-92) and a Lectureship in Virology (1992-97), since 1997, he has been a Senior Lecturer at the University of Edinburgh. Since 2008, he has been a Group Leader/Senior Research Fellow in The Division of Infection and Immunity at The Roslin Institute.
Dr Dalziel’s research interests focus on the host: virus interactions in herpesvirus infections of animals and humans. He has previously investigated the biology of VZV latency and mechanisms of induction of post-herpetic neuralgia (PHN) and the role of virus encoded miRNAs in herpesvirus infection. Current work focuses on applying genome editing approaches to studying herpesvirus biology. His group has developed Genome-Scale CRISPR Knock-Out (GeCKO) and Synergistic Activator Mediator (SAM) libraries directed against the bovine genome. Using these approaches his group has identified cellular genes that control the replication of BoHV-1, the causative agent of Infectious Bovine Rhinotracheitis.
He is the co-author of the 6th edition of Mims Pathogenesis of Infectious Disease (2015), and Editor in Chief of Veterinary Research Communications.
Robert R. Webb PhD is a co-founder and a member of the Board of Directors of Manzanita Pharmaceuticals, Inc.
He is currently retired from the Pharmaceutical and Biotech industries, where as a Medicinal Chemist, he guided and participated in several discovery and development programs that yielded marketed drugs in the anti-infective and cardiovascular fields. Dr. Webb was also a co-inventor on key first-generation Manzanita patents that led to the development of the Company’s current products and technologies. He currently serves as founder and board member for a technology start-up, Vozzi.
Gary Blair, MD
Until his retirement in 2016, Dr Blair was a Board Certified Emergency Medicine Physician with a long-standing interest in finding effective non-narcotic alternatives to pain management for his patients.
Steven J. Borst
Steve Borst has 26 years of operational and venture capital experience, and is currently the Vice President of Finance and Corporate Development for Q Therapeutics, Inc., a venture backed start-up developing cell-based therapies for degenerative conditions of the brain and spine. (www.qthera.com) Prior to joining Q, Steve was a General Partner of Utah Ventures, an early-stage healthcare and information technology fund in Salt Lake City. He has co-founded four venture-backed life science companies in his career and worked with both Frontenac Company and Capital Health Venture Partners. He has a B.S. degree in Industrial Engineering and Operations Research from the University of Michigan and an MBA from the J.L. Kellogg Graduate School of Management at Northwestern University.
Christopher J. Dunn, MD, MBA, FCCP, Observer, Board of Directors
Dr. Chris Dunn serves as Observer to the Manzanita Pharmaceuticals, Inc. Board of Directors. He has over 30 years’ experience as a physician, entrepreneur, and investor where he has been involved with patient care and administration in hospital, medical office and sub-acute settings; in the medical transportation industry, where he served as the Medical Director for Northern California Critical Care Transport for the ambulance company AMR for 20 years; and has served as a physician in the U.S. Air Force Reserves.
Dr. Dunn has provided patient care in pulmonary medicine, internal medicine and critical care medicine at Samaritan House (Redwood City, CA, 2001 to present); in Pulmonary and Critical Care Medicine at Sequoia Hospital (Redwood City, CA 1998-2001); and at Palo Alto Medical Clinic (Palo Alto, CA, 1987) and at Mills-Peninsula Hospital (San Mateo, CA 1984-1987). Complementing his work at Samaritan House, he has participated in medical mission work through his local church in Africa, Middle East, Latin America, and South America.
Since joining Life Science Angels (LSA) in 2005, Dr. Dunn has made over 25 early-stage life science investments with LSA and other angel groups including Mighty Capital LLC. He has acted as due diligence lead and/or founding Board member for early-stage investments in novel medical devices and therapeutics; operations technology for supply and drug distribution; and computerized health records.
Dr. Dunn earned his BA in Biology, a Masters of Health Services Administration and his MD at Stanford University and Stanford School of Medicine. He completed his residency in Internal Medicine at Loma Linda University Medical Center, then served at Loma Linda first as Chief Resident, Internal Medicine and then as a Fellow in Pulmonary and Critical Care Medicine. In 2001, Dr. Dunn earned his MBA from Santa Clara University.
Since 2001, Dr. Dunn has served as a Colonel in the U.S. Air Force Reserves, 349th Air Mobility Wing, Medical Squadron at Travis Air Force Base (Travis, CA). He is trained in Critical Care Air Team Transport (CCATT) and has served as a flight surgeon for the 349th, as Chief of Flight Medicine (2003-2005) and as Chief of Hospital Services (2005-2009). As a CCAT physician, he served in Operation Iraqi Freedom (OIF) and Operation Enduring Freedom (OEF) in 2003, 2006 and 2008. In 2006 he was deployed to Balad Air Force Theater Hospital (near Baghdad, IQ) as Medical Director of the Intensive Care Unit. In 2008, Dr. Dunn served as CCATT commander for all CCATT services in Europe and the Middle East.
Scientific Advisors
G. Craig Hill, PhD
Dr. Hill served as Advisor under the company’s completed Department of Defense grant and the ongoing SBIR Phase II grant from the National Cancer Institute. He is a co-inventor of most of the Manzanita issued patents and patents pending. Dr. Hill was the Principal Scientist who reduced to practice the company’s bioconjugation strategies for the neurotrophin-targeted glucocorticoid and dyes (patents pending).
Dr. Hill completed his undergraduate studies at Cal Poly San Luis Obispo in Biochemistry (1986), earned his PhD in Medicinal Chemistry at the University of Arizona (1990), and served as NIH Postdoctoral Fellow at University of Texas at Austin with Professor Lawrence Hurley. He has previously served as a Medical Affairs Scientist at Leidos Biomedical Research Inc. at the Frederick National Laboratory for Cancer Research in direct support of the Cancer Imaging Program, National Cancer Institute, National Institutes of Health. At LBRI, Dr. Hill coordinated PET development projects for the diagnosis of cancer involving the bioconjugation of monoclonal antibodies with bi-functional chelating agents and their subsequent binding to radio-metals, small molecule radio-labeling, filing of their INDs, monitoring of their clinical trials, and coordination of the bioinformatics development of TCGA and CPTAC imaging efforts into The Cancer Imaging Archive.
He is currently a Radiochemist for SpectronRx, a CMO producing advanced targeted Radio-Immunotherapy Therapy (RIT) cancer drugs with leading international pharmaceutical companies.
Robert B. Campenot, PhD (Emeritus)
Bob Campenot joined Asilomar as an advisor in 1998 and continued to serve as an advisor to Manzanita until his retirement in 2015.
Dr. Campenot has been a member of the Faculty of Medicine and Dentistry of the University of Alberta since 1987, and has served as Full Professor in the Department of Cell Biology since 1992. A US citizen, prior to coming to Alberta, Dr Campenot was an Assistant Professor in the Department of Neurobiology and Behavior at Cornell University. He holds a BA in Psychology from Rutgers University, an MS in Physiology from UCLA, and a PhD in Biological Oceanography from the Woods Hole Oceanographic Institution/MIT joint program (1976). He was a postdoctoral fellow in the Neurobiology Department of Harvard Medical School.
Dr Campenot’s research interests were in neuronal development, and nerve growth and regeneration. His work involved cultured neurons analyzed primarily with biochemical methods. Major research accomplishments included invention of the compartmented culture technique (Campenot, 1977, Proc. Natl. Acad. Sci. USA 74, 4516) and the subsequent characterization of many important principles of axonal growth and axonal and neuronal survival. He used compartmented cultures to accomplish the first quantitative study of NGF retrograde transport and processing (Ure and Campenot, 1997, J. Neurosci. 17, 1282) and the first study of retrograde phosphorylation signaling to cell bodies (Senger and Campenot, 1997, J. Cell Biol. 138, 411).
Dr Campenot’s work showed that survival signals to the cell body initiated by NGF at axon terminals could be transmitted unaccompanied by NGF transport, findings that contradicted a 30-year dogma (MacInnis and Campenot, 2002, Science 295, 1536). Work from Dr. Campenot’s laboratory also showed that NGF-deprived axons generate a retrograde death signal that is transported to the cell bodies (Mok, Lund, and Campenot, 2009, Cell Research). This was the first time that death signaling from axons, rather than survival signaling, was implicated in the control of neuronal survival by neurotrophins. Other work provided evidence that slow axonal transport of tubulin and other cytosolic molecules may be accomplished by carrying these molecules on the surfaces of fast transport vesicles (Campenot et al., 2003, Neuropharmacol. 44, 1107). The compartmented culture system is recognized as the best model system for studies of retrograde signaling and has been widely adopted other research groups.
